|
GASTON PLANTE LEAD ACID BATTERY INVENTOR
|
|||
|
GASTON
PLANTE - The obituary of the battery inventor in a Paris newspaper
in 1889.
Gaston Planté (22 April 1834 – 21 May 1889) was the French physicist who invented the lead–acid
battery in 1859. The lead-acid battery eventually became the first rechargeable electric battery marketed for commercial use.
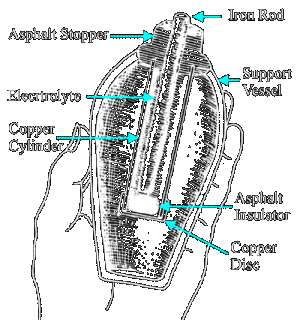
BAGHDAD BATTERY - The first battery is believed to date from 248 BC: The Bagdad Battery was built in the Parthian or Sassanid period ~248 BC - 226 AD. The battery consisted of a carbon rod in the center of a clay (earthenware) vase. The rod was surrounded by an unknown electrolyte (likely to be orange/lemon juice), then copper, then asphaltum. Each battery had a weight of about 2 kilograms and produced 0.4 - 0.5 volts with open contacts. These batteries were very weak. The "Bagdad Battery" was found in 1936 and is believed to be authentic by many reputable sources.
One
of these batteries rests in the National Museum of Iraq is a jar about the size of a man's fist.
The little jar in Baghdad suggests that Volta reinvented the battery but
of course had no knowledge of ancient history to go on. The jar was first described by
German archaeologist Wilhelm Konig in 1938. It is unclear if Konig dug the object up himself or located it within the holdings of the museum, but it is known that it was found, with several others, at a place called Khujut Rabu, just outside Baghdad.
THE
LEAD-ACID BATTERY (ACCUMULATOR)
ALLESANDRO VOLTA - The "Voltaic pile", a stack of zinc and silver disks separated by a wet cloth containing a salt or a weak acid solution, was the first battery known to Western civilization. The important part of the equation being more than one cell in combination to form a battery of cells, whereas the Baghdad battery is more accurately described as a cell. Of course today we describe AA and AAA size single cells as batteries.
Alessandro Giuseppe Antonio Anastasio Volta (Italian: [alesˈsandro ˈvɔlta]; 18 February 1745 – 5 March 1827) was an Italian physicist, chemist, and a pioneer of electricity and power, who is credited as the inventor of the electrical battery and the discoverer of methane. He invented the Voltaic pile in 1799, and reported the results of his experiments in 1800 in a two-part letter to the President of the Royal Society. With this invention Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments which eventually led to the development of the field of electrochemistry.
AUSTIN HOPKINSON MP
Austin
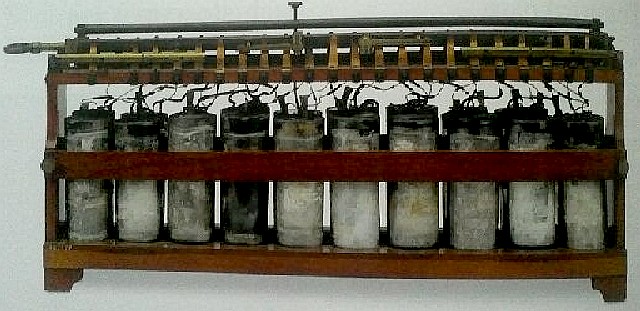
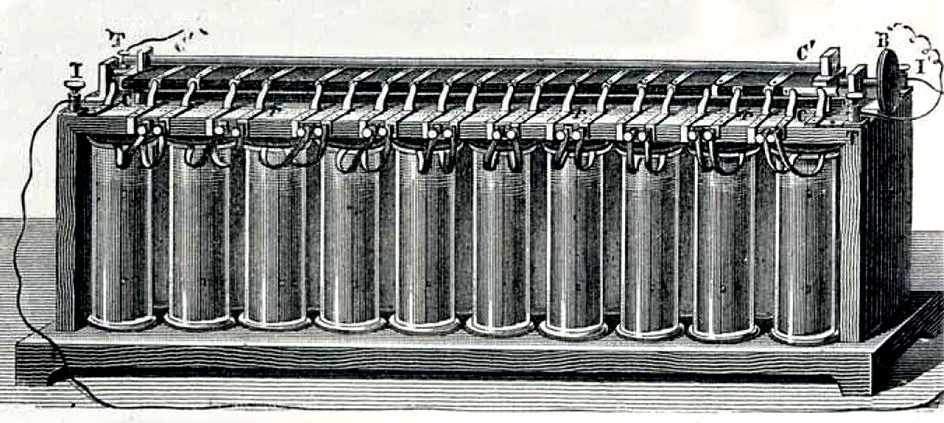
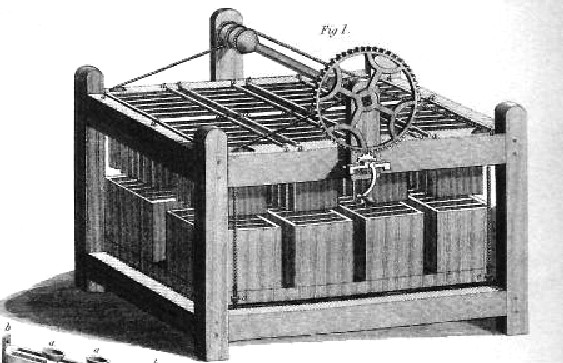
A BATTERY OF CELLS - Gaston Plante(1834-1889), while working in Paris invented this lead-acid ancestor of today’s car battery. Lead-acid batteries remain the commonest type of vehicle battery to this day.In 1868 French chemist Georges Leclanche created the first “wet cell” battery also called as Leclanche cell.
A battery is made of two or more cells. Each cell is composed of two different materials with an electrolyte in between. Early engineers discovered that with the right materials, negatively charged ions are attracted to the cathode (-), meanwhile positively charged ions are attracted to the anode(+) (the other electrode). There are many types of batteries and please see the list further down this page.
By 1868 twenty thousand Leclanché cells were being used in telegraph systems. The original Leclanché cells were built in porous pots which were heavy and subject to breakage. Within twenty years other inventors had modified the design into what we now know as "dry cells" which became widely used in the first flashlights (1909) and in battery-powered radios of the 1920s.
A battery converts chemical energy into electrical energy. The battery was the first device developed to power electrical devices, only later on in the mid 1800's did the dynamo and generator take over as a primary power source. Batteries still occupy an indispensable role everywhere in our lives. They come in all shapes and sizes. Today's engineers are working on exciting advancements, mainly improving energy density and recharge speeds.
Improvements in batteries result in very visible changes in society because often it is often battery size, weight and cost which are limiting factors in the advancement of a technology. There are many ways to make a battery and some using carbon nanotubes are being advanced as fast as researchers can go today, looking for ways to improve the energy density and longevity for electric cars and trucks.
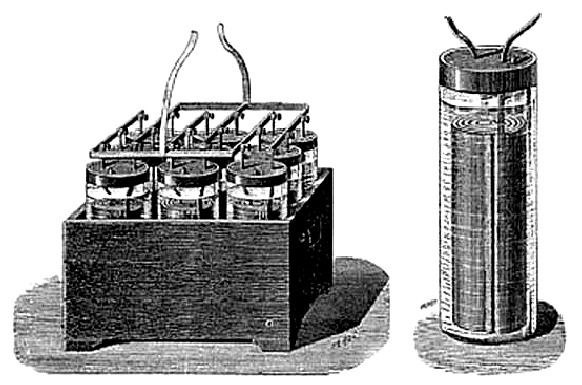
LECLANCHE
BATTERY - This handsome, six-element plunge battery is in the Jack Judson Collection at the Magic Lantern Museum in San Antonio, Texas. It is marked "C.H. Stoelting Co." of Chicago.
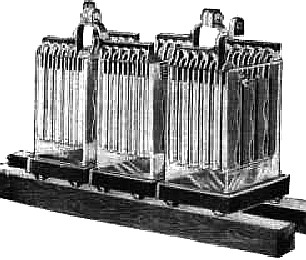
MORE RAW STORAGE - For energy storage at Herstmonceux more grunt was needed to be able to light the manor house and the houses in the village, to include the street lighting. Accumulators like that seen above were placed in rows on timber beams balanced on circular glass supports that we have buckets of in the museum. These small bowl-like glass supports allowed the underside of the battery trays to breathe and stop condensation. Many of these were dug up on site during the restorations. Modern batteries, such as the SBS brand seen above, use similar construction and glass casings and have a claimed life-span of up to 20 years, compared with the life of a car battery that is around 3 to 5 years for the better made units.
NIFE 1905 - Thomas Edison, who was as much a chemist as an all-around inventor, thought that the lead in Planté-type cells made them too heavy, and that having acid in contact with any metal was an inherently bad idea. After much experimentation, he developed a successful alkaline battery. The Edison cell uses an iron anode, nickel oxide cathode, and KOH electrolyte. This cell is extremely rugged and is still used in certain industrial applications and locomotives, but it was never able to displace the lead-acid cell as Edison had hoped.
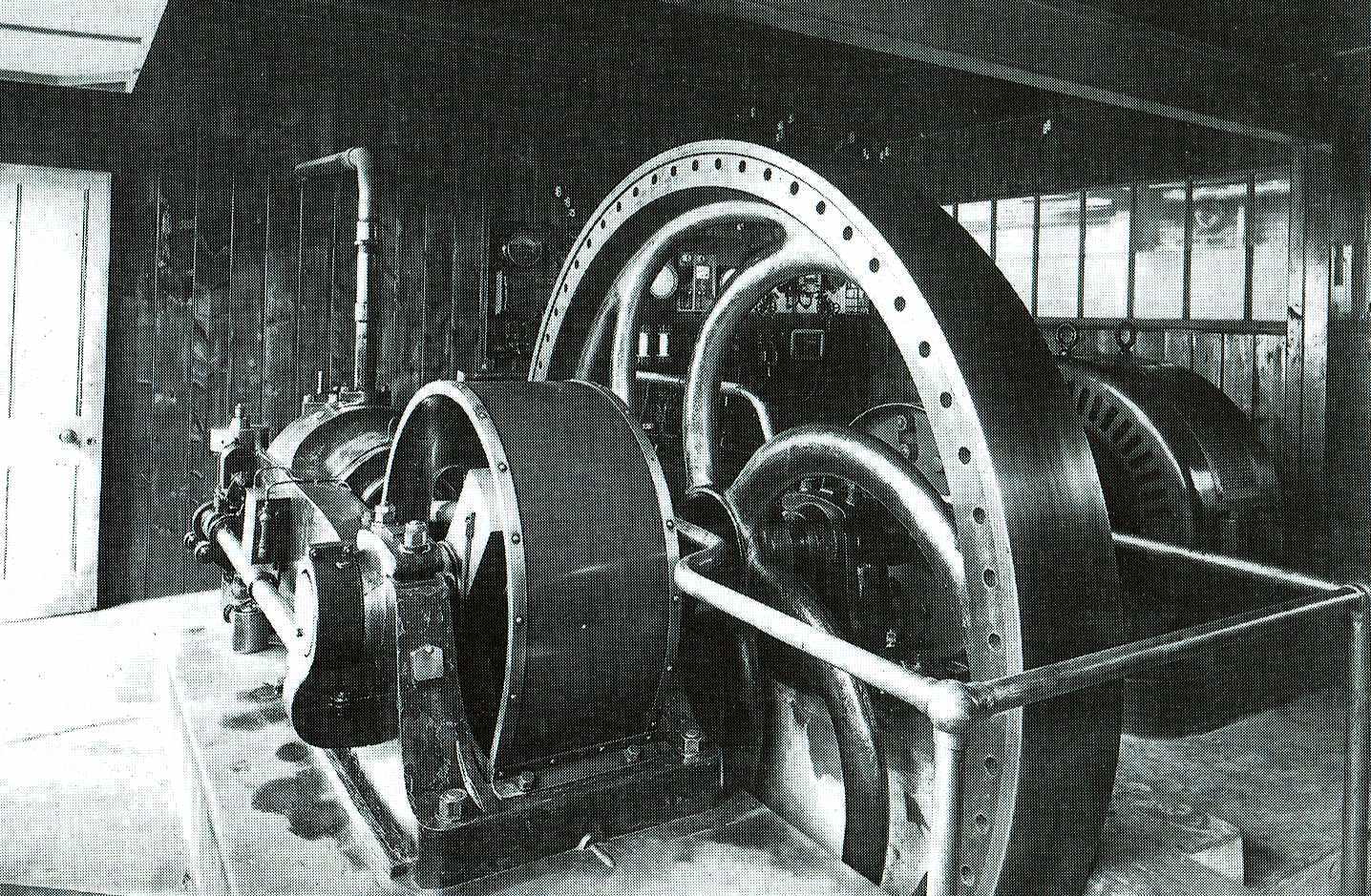
GAS ENGINE - Mounted on substantial concrete bases and secured by large steel bolts, this is a picture of a generator that did the rounds in Sussex, finally ending up powering a tram in Eastbourne in the 1960s, but not before being used to drive machinery for the Eastbourne Aviation Company between 1911 and 1924. This generator set is virtually identical to the one that was situated in Herstmonceux Museum around 1909, identified by the concrete base footprint. This is a 36 horsepower National unit mated to a Crompton dc machine of 110/220 volts. Charles de Roemer (von Roemer) the installer of these units, is known to have had an association with John Hopkinson and possibly also Thomas Edison, because of the litigation between Joseph Swan and the American inventor.
TYPES
OF BATTERY - PRIMARY & SECONDARY CELLS
BATTERY BANK - The old generating rooms just outside of Herstmonceux village in Lime Park. The building on the left was the battery store. It is now a domestic store. The skylights were a necessary feature to ventilate the battery and generating rooms of fumes from the sulfuric acid when they charged which gave off an explosive gas.
This picture was taken some years ago when the Second World War covering of corrugated iron was first removed from the north facing elevation. Since then the perforated iron roof has been replaced with slate and vehicles on display in the grounds have been housed, along with other improvements that include solar water heaters and photovoltaic panels to provide heat and electricity from nature to be harvested for a sustainable future.
HERITAGE INDEX A - Z
AVIATION - EASTBOURNE BARCLAYS BANKING LET DOWN - MISSING ACCOUNT MONEY BARON CARL VON ROEMER & CHARLES de ROEMER CAMPBELL HALL - BLUEBIRD ELECTRIC CARS GAS ENGINES - COAL CONVERSION, INTERNAL COMBUSTION OBSERVATORY - HERSTMONCEUX CASTLE SOLAR LADY - STATUE
LINKS & REFERENCE
https://en.wikipedia.org/wiki/John_Hopkinson https://en.wikipedia.org/wiki/Edward_Hopkinson https://en.wikipedia.org/wiki/Austin_Hopkinson https://en.wikipedia.org/wiki/Alfred_Hopkinson
|
|||
|
This website is Copyright © 2023. All rights reserved. All other trademarks are hereby acknowledged. Contact Us www.cherrymortgages.com
|